For the first time, scientists observed oxygen-13 undergoing a unique radioactive decay, producing three helium nuclei, a proton, and a positron, using advanced equipment at Texas A&M University’s Cyclotron Institute.
The Science
Not all of the material around us is stable. Some materials may undergo radioactive decay to form more stable isotopes.
Scientists have now observed a new decay mode for the first time. In this decay, a lighter form of oxygen, oxygen-13 (with eight protons and five neutrons), decays by breaking into three helium nuclei (an atom without the surrounding electrons), a proton, and a positron (the antimatter version of an electron).
Scientists observed this decay by watching a single nucleus break apart and measuring the breakup products.
The Impact
Scientists have previously observed interesting modes of radioactive decay following the process called beta-plus decay. This is where a proton turns into a neutron and emits some of the produced energy by emitting a positron and an antineutrino. After this initial beta-decay, the resulting nucleus can have enough energy to boil off extra particles and make itself more stable.
This new decay mode is the first observation of three helium-nuclei (alpha particles) and a proton being emitted following beta-decay. The findings can inform scientists about decay processes and the properties of the nucleus before the decay.
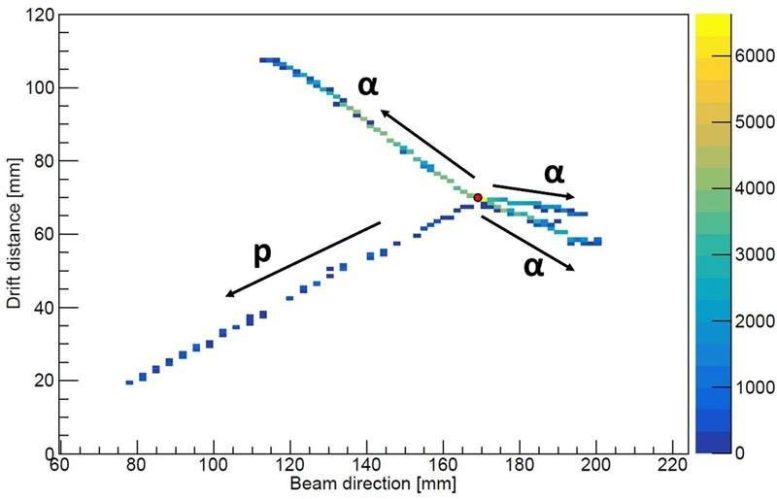
Image of particles that have emerged from the nucleus after it undergoes beta decay from this new decay mode. The resultant nucleus has broken apart into three helium nuclei (α) and one proton (p) originating from a single decay point (red circle). Credit: Image courtesy of J. Bishop
Summary
In this experiment, researchers used a particle accelerator known as a cyclotron at the Cyclotron Institute at Texas A&M University to produce a beam of radioactive nuclei at high energies (approximately 10% the speed of light). They sent this beam of radioactive material, oxygen-13, into a piece of equipment known as the Texas Active Target Time Projection Chamber (TexAT TPC). The material stops inside this detector, which is filled with carbon dioxide gas, and decays after about ten milliseconds by emitting a positron and a neutrino (beta-plus decay).
By implanting the oxygen-13 into the detector one nucleus at a time and waiting for it to decay, the researchers measured any particles that boil off following the beta-decay using the TexAT TPC. Next, they analyzed the data with a computer program to identify the tracks the particles leave in the gas. This allowed them to identify the rare events (occurring only once per 1,200 decays) as those where four of the particles are emitted following beta-decay.
 Reviewed by Team
on
September 16, 2023
Rating:
Reviewed by Team
on
September 16, 2023
Rating:





No comments: